Chapter 4: Infection prevention and control
Skip chapter table of contents and go to main content
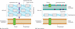
Anatomy and physiology
Pathogens are what cause infection. It is important to understand types of pathogen, how they spread and what kinds of environment are favourable for their growth so that effective infection prevention and control measures can be put in place.
Causes of infection
The term ‘infectious agent’ is often used to describe anything that may be transmitted from one person to another, or from the environment to a person, and subsequently cause an infection or parasitic infestation.
Distinct types of infectious agent act differently and have different impacts on the infected individual. For example, whether a particular infectious agent will cause an infection in any given circumstance is dependent on many factors, including how easily that agent can be transmitted, its pathogenicity (its ability to cause disease) and its virulence (the severity of the infection produced) (Gillespie and Bamford [45]). The susceptibility of the patient to infection is also a significant influence.
To practice effective infection prevention and control, it is helpful to understand the ‘chain of infection’ (Damani [17]). This is a helpful model to use when considering how infection can be prevented, as it shows how it is possible to break the ‘links’ in the chain. For an infection to exist, there must be an organism (pathogen) and it must be able to get into a susceptible host, multiply and exit. It may need a place to hide (reservoir) while waiting for the next susceptible host. Figure 4.1 illustrates the chain of infection and Table 4.1 lists the links with examples of how infection can be prevented at each link. The major groups of micro‐organisms are described below.
Table 4.1 Links in the chain of infection
| Link | Definition | Example | Examples of breaking the chain |
|---|---|---|---|
| Infectious agent | A potentially pathogenic micro‐organism or other agent |
|
|
| Reservoir | Any location where micro‐organisms hide, exist or reproduce |
|
|
| Portal of exit | The route by which the infectious agent leaves the reservoir |
|
|
| Mode of transmission | The way the infectious agent is spread (see definitions section above) |
|
|
| Portal of entry | The route by which the infectious agent enters a new host |
|
|
| Susceptible host | The person that the infectious agent enters has to be susceptible to infection |
|
|
Types and classification of micro‐organisms
Historically, the classification of micro‐organisms was based on physical characteristics such as their size, shape or ability to retain a particular stain to make them visible under the microscope. Some of these distinctions are still useful, but classification is increasingly based on genetic characteristics, as increasingly sophisticated analysis techniques (such as genomic sequencing) reveal the actual relationships between organisms. This can lead to confusion as new discoveries lead to species being reclassified and renamed. For example, ‘methicillin‐resistant’ Staphylococcus aureus is now ‘meticillin‐resistant’ and Clostridium difficile is now termed Clostridioides difficile.
It should also be noted that there can be a wide variety of characteristics within each species, leading to significant variations in the severity of infection caused by different strains of the same organism. An example of this is Group A Streptococcus pyogenes, which is a common cause of sore throat but can also cause skin conditions such as erysipelas, scarlet fever, toxic shock syndrome and necrotizing fasciitis. Another is Escherichia coli, which is carried in the gut of all mammals with no ill effects but whose toxin‐producing O157:H7 strain can cause serious illness.
In printed text, the names of bacteria are written in italics, with the name of the genus capitalized and the species in lower case, for example Staphylococcus aureus. The abbreviation ‘spp.’ is used to refer to all of the species of a genus, for example Klebsiella spp. This section gives an overview of the different types of organism that may be encountered in a healthcare environment as well as the differences between and within the types.
Bacteria
Bacteria are probably the most important group of micro‐organisms in terms of infection prevention and control because they are responsible for the majority of opportunistic infections in healthcare. A healthy human being will typically be host to a quadrillion (1000 trillion or 1015) bacteria – around ten times as many organisms as there are cells in the human body – and we need most of these to survive.
The so‐called ‘human microbiome’ is increasingly being recognized as an essential part of human health (Bhalodi et al. [7], Young [132]) and a variety of conditions – such as Crohn's disease, ulcerative colitis, irritable bowel syndrome, obesity, type 2 diabetes, Parkinson's disease, chronic fatigue syndrome, arthritis and even asthma – may all be related to disturbance of the balance of micro‐organisms in the gut, although the question remains as to whether this is a cause or effect (Otter [90], Tosh and McDonald [113], Wang et al. [120]). See Table 4.2 for examples of how the human microbiome can be protective.
Table 4.2 Examples of how the human microbiome can be protective
| Bacteria | Comments |
|---|---|
| Gut flora including Bacteroides spp., Bifidobacterium spp., Enterobacter spp., Klebsiella spp., Enterococcus spp. and Escherichia coli |
Disturbance though antibiotics, surgery or chemotherapy may have far‐ranging effects on the human body, including obesity, inflammatory bowel diseases, antibiotic‐associated diarrhoea and cancer. |
| Skin flora including Staphylococcus epidermidis, Staphylococcus aureus, diphtheroids and Candida spp. | A healthy, intact, normal resident skin flora means that pathogenic organisms are less likely to settle on the skin and cause infection. |
| Vaginal flora including Lactobacillus spp. and diphtheroids | Babies born per vagina are more likely to have their skin colonized with the ‘right’ organisms, which reduces problems with skin and allergies. |
In normal circumstances, the relationship between bacteria and their host is symbiotic and the organisms are considered to be commensal (i.e. their presence does not cause the host any problems) and mutually beneficial; however, if the host has lowered resistance or a bacteria gains access to a different site, it can become an opportunistic pathogen. For example, E. coli from the gut may cause a urinary tract infection (with associated symptoms) if it enters the urethra and ascends the urinary tract.
Despite the fact that we are surrounded by unquantifiable numbers of bacteria in our world, relatively few are pathogenic to us. There is an important balance to be struck in our home lives; we should not try to disinfect everything we come into contact with, and indeed many things around us are going to be contaminated (money, cash point buttons, our mobile phones and the handles on public transport, to name a few). If we are healthy and have good immunity and intact skin, this will often be of little consequence to us as long as we follow simple precautions such as practising hand hygiene, environmental hygiene (cleaning) and food hygiene.
For a patient receiving interventional healthcare, however, things can be very different and we need to do as much as possible to ensure items introduced into the care environment are free of pathogens. Our increasing understanding of the normal commensal micro‐organisms in humans suggests that restoring and maintaining the microbiome may provide a key to preventing colonization and infection, including with multi‐drug‐resistant organisms (Otter [90], Tosh and McDonald [113]), which can be ‘selected out’ when exposed to antibiotics. This means that bacteria that are sensitive to the antibiotics are killed but any resistant ones are left to replicate and become the dominant type. A developing form of treatment is the ‘faecal microbiota transplant’, or stool transplant, which involves replacing the stool in an affected gut with stool from a healthy donor. This has been shown to be very effective for treatment of intractable C. difficile (van Nood et al. [115]) and may be helpful in other conditions.
Sometimes a patient will be ‘colonized’ with a species of bacteria, which means it is present but not causing them harm. However, if the bacteria are transferred to another patient and gain access to a portal of entry, that person may suffer harm, so there is a need for effective precautions. Whether or not any particular situation will result in an infection depends on a wide range of factors and these are not always predictable. What is certain is that bacterial infections cannot occur when bacteria are not present, hence the importance of measures designed to minimize the risk of transmission.
The presence of an organism in a specimen result does not on its own imply that an infection has occurred. Any laboratory results must always be interpreted in association with an assessment of the patient's condition and symptoms, which will guide the need for treatment.
Morphology
Bacterial cells are much smaller and simpler than human cells; this small size means that bacteria do not have separate structures (such as a nucleus) within their cells. The structure of the cell wall determines another important distinction in medically significant bacteria: whether they are gram positive or gram negative. The ‘gram’ in these terms refers to Gram staining, named after its Dutch inventor, Hans Christian Gram (1853–1938), who devised the stain in 1884. The structure of the cell wall determines whether or not the bacteria are able to retain a particular stain in the presence of an organic solvent such as acetone. This structure also determines other characteristics of the bacteria, including their susceptibility to particular antibiotics, so knowing whether the cause of a bacterial infection is ‘gram positive’ or ‘gram negative’ can help to determine appropriate treatment (Goering et al. [47]). The structures of the two different types of cell wall are shown in Figure 4.2.
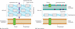
Figure 4.2 (a) Gram‐positive and (b) gram‐negative bacterial cell walls.
Source: Adapted from Elliot et al. ([38]) with permission of John Wiley & Sons.
Other structures visible outside the cell wall may include pili, which are rigid tubes that help the bacteria attach to host cells (or, in some cases, other bacteria for the exchange of genetic material); flagellae, which are longer, mobile projections that can help bacteria to move around; and capsules, which can provide protection or help the bacteria to adhere to surfaces. These are illustrated in Figure 4.3. The presence or absence of different structures plays a part in determining an organism's pathogenicity – that is, its ability to cause an infection and the severity of that infection (Goering et al. [47]).
A final bacterial structure to consider is the spore. Bacteria reproduce via a process called ‘binary fission’ – they create a copy of their genetic material and split themselves in two, with each ‘daughter’ cell being an almost exact copy of the ‘parent’ (there are mechanisms by which bacteria can transfer genetic material between cells and so acquire characteristics such as antibiotic resistance, but they are beyond the scope of this chapter). Some bacteria, notably the Clostridia, have the capacity, in adverse conditions, to surround a copy of their genetic material with a tough coat called a ‘spore’. Once the spore has been formed, the parent cell dies and disintegrates, leaving the spore to survive until conditions are suitable for it to germinate into a normal, ‘vegetative’ bacterial cell, which can then reproduce (Goering et al. [47]). Spores are extremely tough and durable. They are not easily destroyed even by boiling or via the alcohol‐based handrubs widely used for hand hygiene, hence the need to physically remove them from the hands by washing with soap and water when caring for a patient with C. difficile infection. Commonly used disinfectants containing quaternary ammonium compounds (such as benzalkonium chloride) are ineffective against spores.
Some bacteria produce toxins, which are proteins released by the bacteria that can increase the severity of disease. Endotoxins are pieces of the cell wall of gram‐negative bacteria; these initiate a strong immune response from the body, which can cause catastrophic damage. For example, endotoxins of Neisseria meningitidis cause the breakdown of blood vessels, leading to anoxic tissue and the need for amputation. Antibiotics may kill the bacteria but in doing so flood the body with deadly endotoxins.
Some medically significant bacteria are listed in Table 4.3. A few bacteria do not easily fit into the gram‐positive/negative dichotomy. The most medically significant of these are the mycobacteria, which have a waxy coat and are responsible for diseases including tuberculosis and leprosy (Goering et al. [47]).
Table 4.3 Medically significant bacteria
| Spherical | Rod‐shaped | |
|---|---|---|
| Gram positive |
Staphylococcus aureus
Streptococcus spp. |
Clostridioides difficile
Clostridium tetani
Bacillus spp. |
| Gram negative |
Neisseria meningitidis
Neisseria gonorrhoeae
|
Escherichia coli
Pseudomonas aeruginosa
Klebsiella pneumoniae
Acinetobacter baumannii
Salmonella spp.
Legionella pneumophila
|
Culture and sensitivity testing
When a sample arrives in the laboratory, it is put onto agar plates to culture any organisms present. The site of the specimen and clinical information may dictate which tests are deployed and what media are used, which is why it is very important to fill out the microbiology request form with as much detail as possible (for more detail see Chapter c13: Diagnostic tests). The provision of accurate and comprehensive information assists the microbiologist in interpreting the findings in the laboratory and simple information, such as the site of the specimen (if a wound), the type of urine, recent travel and current antibiotic use, can also be helpful.
Different types of agar plate may be used to grow different bacteria. Once the organism has been grown, it can be subjected to further tests to identify it, including a gram stain to see whether it is gram positive or gram negative, examination for the presence of pus cells and sensitivity testing. Sensitivity testing usually involves spreading the organism over an agar plate that contains small antibiotic discs. If the bacteria grow all the way up to the disc, they are resistant to that antibiotic. A ‘zone of inhibition’ around the disc implies they are sensitive to the antibiotic and that the antibiotic may be used to treat that infection. A faster and more modern technique to identify and speciate microbes involves the use of matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) (Croxatto et al. [14]).
Modern laboratories use molecular technology to diagnose patients without the need for culture. These techniques include polymerase chain reaction (PCR) and enzyme immuno‐assay.
Viruses
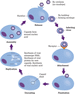
Viruses are much smaller, and even simpler, than bacteria. Nobel laureate Peter Medawar is said to have described viruses as ‘bad news wrapped in protein’ and indeed they are little more than a protein capsule containing some genetic material. They rely on other organisms for their survival and reproduce within a host cell, using the cell's own mechanisms to reproduce, which leads to the death of the host cell (Goering et al. [47]). The life cycle of a virus is illustrated in Figure 4.4. The small size of viruses (e.g. poliovirus is only 30 nanometres across) means that most are smaller than the wavelengths of visible light. They can only be ‘seen’ with a specialist instrument such as an electron microscope, which will only be available in a very few hospital microbiology laboratories. Diagnosis of viral infections is normally based on the patient's symptoms, with confirmation by laboratory tests designed to detect either the virus itself or antibodies produced by the patient's immune system as a response to infection (Goering et al. [47]). Modern laboratories use PCR to amplify the genes in the sample to make them detectable quickly.

Figure 4.4 The viral life cycle. RNA, ribonucleic acid.
Source: Adapted from Perry ([93]) with permission of John Wiley & Sons.
There are viruses that specifically infect humans, other animals or plants, or even bacteria. This is one characteristic that can be used in classifying them. However, the main basis for classification is by the type of genetic material they contain – DNA (deoxyribonucleic acid) or RNA (ribonucleic acid), in either a double or single strand. Other characteristics include the shape of the viral particle and the sort of disease caused by infection (Gillespie and Bamford [45]).
A final point to consider in relation to viral structure and infection prevention and control is the presence or absence of a lipid envelope enclosing the viral particle. Viruses that have a lipid envelope, such as herpes zoster virus (responsible for chickenpox and shingles), are much more susceptible to destruction by alcohol than those without. Norovirus and or rotavirus, which are common causes of viral gastroenteritis (WHO [124]), are examples of viruses without a lipid envelope. For this reason, alcohol hand sanitizers are not recommended during outbreaks of norovirus in hospitals.
Fungi
Like bacteria, fungi exist in many environments on earth, including occasionally as commensal organisms on human beings. Fungi are familiar to us as mushrooms and toadstools and the yeast that is used in brewing and baking. They also have many uses in the pharmaceutical industry, particularly in the production of antibiotics. Fungi produce spores, both for survival in adverse conditions, as bacteria do, and to provide a mechanism for dispersal in the same way as plants (Goering et al. [47]).
A few varieties of fungi are able to cause opportunistic infections in humans. These are usually found in one of two forms: either as single‐celled yeast‐like forms, which reproduce in a similar fashion to bacteria (by dividing or budding), or as plant‐like filaments called ‘hyphae’. A mass of hyphae together forms a ‘mycelium’. Some fungi may appear in either form, depending on environmental conditions. Fungal infections are referred to as ‘mycoses’. Superficial mycoses, such as ringworm and thrush (Candida albicans), usually involve only the skin or mucous membranes and are normally mild, if unpleasant; however, deeper mycoses involving major organs can be life threatening. These occur in patients who have severely impaired immune systems and may be an indicator of such impairment; for example, pneumonia caused by Pneumocystis jirovecii (previously carinii) is considered a clinical indication of AIDS (acquired immune deficiency syndrome). Superficial infections are generally transmitted by physical contact, whereas deeper infections can result from spores being inhaled. This is why it is important to ensure that patients with impaired immunity are protected from situations where the spores of potentially pathogenic fungi, such as Aspergillus spp., are likely to be released, for example during building work (Goering et al. [47]).
Protozoa
Protozoa are single‐celled animals, some species of which are medically important parasites of human beings, particularly in tropical and subtropical parts of the world, where diseases such as malaria are a major public health issue. Unlike bacteria, their relationship with humans is almost always parasitic. The life cycles of protozoa can be complex and may involve stages in different hosts.
Medically important protozoa include Plasmodium spp., the cause of malaria; Giardia spp. and Cryptosporidium spp., which can cause gastroenteritis; and Trichomoniasis spp., which is a sexually transmitted cause of vaginitis (Gillespie and Bamford [45]).
The most common routes of infection of protozoa are by consuming them in food or water or via an insect vector such as a mosquito (Goering et al. [47]). Cross‐infection in the course of healthcare is uncommon but not unknown.
Helminths
‘Helminths’ is a generic term for parasitic worms. A number of worms from three different groups affect humans: tapeworms (cestodes), roundworms (nematodes) and flukes (trematodes). Transmission generally occurs via ingestion of eggs or larvae, or infected animals or fish, but some are transmitted via an insect vector and some, notably the nematode Strongyloides spp., have a larval stage that is capable of penetrating the skin (Gillespie and Bamford [45]).
Helminth infections can affect almost every part of the body, and the effects can be severe. For example, Ascaris worms can cause bowel obstruction if there are large numbers present; Brugia spp. and Wuchereria spp. obstruct the lymphatic system and eventually cause elephantiasis as a result; and infection with Toxocara spp. (often after contact with dog faeces) can result in epilepsy or blindness (Goering et al. [47]). However, cross‐infection in healthcare is not normally considered a significant risk.
Arthropods
Arthropods (insects) are most significant in infectious disease in terms of their function as vectors of many viral, bacterial, protozoan and helminth‐caused diseases. Some flies lay eggs in the skin of mammals, including humans, and the larvae feed and develop in the skin before pupating into the adult form, whereas some, such as lice and mites, are associated with humans for the whole of their life cycle. Such arthropod infestations can be uncomfortable, and there is often significant social stigma attached to them, possibly because the creatures are often visible to the naked eye. The activity of the insects and the presence of their saliva and faeces can result in quite severe skin conditions that are then vulnerable to secondary fungal or bacterial infection (Goering et al. [47]).
Lice
Species of Pediculus infest the hair and body of humans, feeding by sucking blood from their host. The adult animal is around 3 mm long and wingless, moving by means of claws. It cannot jump or fly, and dies within 24 hours if away from its host, so cross‐infection normally occurs via direct contact or transfer of eggs or adults through sharing personal items (Cummings et al. [15]).
Scabies
Scabies is caused by the mite Sarcoptes scabiei, an insect less than 1 mm long that burrows into the top layers of skin. The female mites lay eggs in these burrows and the offspring can spread to other areas of the body. Infestation usually starts around the wrists and in between the fingers because acquisition normally occurs via close contact with an infected individual (e.g. by holding hands). The burrows are visible as a characteristic rash in the areas affected. The skin starts to itch a few weeks after infestation, which is a reaction to the faeces of the mite. A delay in recognition can lead to mass infestation, especially within families or in settings where there is a lot of interpersonal care, such as a nursing home. In immunocompromised hosts and those unable to practise normal levels of personal hygiene, very high levels of infestation can occur, often with thickening of the skin and the formation of thick crusts. This is known as ‘Norwegian scabies’ and is associated with a much higher risk of cross‐infection than the normal presentation.
Scabies is most often associated with long‐stay care settings, but there have been outbreaks associated with more acute healthcare facilities (Cassell et al. [12]). Treatment with scabicide must be co‐ordinated to ensure untreated hosts do not reinfect those already treated.
Prions
Prions are thought to be the causative agents of a group of diseases called transmissible spongiform encephalopathies (TSEs), the most well known of which are Creutzfeldt–Jakob disease (CJD) and its variant (vCJD) (Table 4.4). These are fatal neurodegenerative diseases with a lengthy incubation period (up to 50 years) and no conventional host response, making them difficult to detect.
Table 4.4 Types of transmissible spongiform encephalopathies
| Type | Examples |
|---|---|
| Idiopathic (just happens for no clear reason) |
Sporadic (classical) CJD
Sporadic fatal insomnia |
| Inherited (genetic) |
Familial CJD
Gerstmann–Sträussler–Scheinker syndrome and variants
Fatal familial insomnia |
| Acquired |
Derived from humans:
Derived from bovines: vCJD (diet – meat infected with bovine spongiform encephalopathy) |
| CJD, Creutzfeldt–Jakob disease; vCJD, variant Creutzfeldt–Jakob disease. |
TSEs can be naturally occurring, inherited or acquired (Table 4.4). They are characterized by ‘plaques’ in the brain that are surrounded by holes that give the appearance of a sponge, hence the name. The causative ‘organism’ is a prion, defined in 1982 by Stanley Prusiner as a proteinaceous infectious particle resistant to procedures that modify nucleic acid.
From an infection control perspective, the key point is that prions contain no genetic material; therefore, it can be argued that they are not alive and so cannot be killed. Control is achieved via recognition of risk and physical removal through cleaning procedures. Prions are not affected by routine decontamination processes such as autoclaving or chemical disinfection. This has led to extensive reviews of decontamination procedures in the UK with increased emphasis on effective washing to remove any residual organic material, and on the tracking of instruments to individual patients to facilitate any look‐back exercise. Modern decontamination services are now capable of removing prions from the surface of even complex instruments; however, where risk is identified, single‐use instruments are usually recommended, especially for neurological work.
In the 1990s there was a lot of concern about the emergence of vCJD, which was associated with consumption of contaminated beef from cattle who had bovine spongiform encephalopathy (BSE). With intense input from public health initiatives and what was then called the Ministry of Agriculture, Fisheries and Food, beef was made safe again. However, UK citizens born before 1992 are still considered at risk of vCJD due to its long incubation period.
There were also several cases of CJD associated with contaminated medical products, such as human pituitary hormone, dura mater grafts and medical instruments. For this reason, all patients undergoing surgery should be assessed for risk of CJD by asking the following questions:
- Do you have a blood family member who has suffered from CJD?
- Have you ever received hormones derived from a human pituitary gland (e.g. growth hormone)?
- Have you ever had a corneal transplant or a dura mater graft?
- Have you been told that ‘you may be at risk of CJD for public health purposes’?
A patient with CJD or vCJD is not infectious to other people under routine circumstances so no special precautions are required other than if dealing with cerebrospinal fluid (CSF). A spillage of CSF should be cleaned up with a strong disinfectant such as 10,000 ppm of chlorine.
Sources of infection
An individual may become infected with organisms already present on their body (endogenous infection) or introduced from elsewhere (exogenous infection). The majority of HCAIs are endogenous, hence the importance of procedures such as effective skin decontamination prior to invasive procedures (NHS England and NHSI [82]).
Indicators and effects of infection
Generally, infection is said to have occurred when infectious agents enter a normally sterile area of the body and cause symptoms as a result. There are obvious exceptions (e.g. the digestive tract is not sterile, being home to trillions of micro‐organisms, but many types of infectious gastroenteritis are caused by particular organisms entering this area), but this is a useful working definition. The symptoms of infection are listed below. Not all symptoms will be present in all cases, and it should be noted that many symptoms are caused by the body's response to infection and so may not be present in severely immunocompromised patients (Fishman [39]).
Symptoms of infection
The cardinal signs of inflammation will often be present:
- Heat: the site of the infection may feel warm to the touch, and the patient may have a raised temperature.
- Pain: at the site of the infection.
- Swelling: at the site of the infection.
- Redness: at the site of the infection
- Loss of function: the affected area may not work properly.
In addition, there may be other signs, such as:
- pus
- raised white cells in blood results
- raised C‐reactive protein (CRP) in blood results
- altered blood gases
- feeling of general malaise
- aching joints
- abdominal pain and tenderness
- nausea, diarrhoea and/or vomiting
- oliguria or anuria
- urinary frequency and/or pain on passing urine (strangury)
- confusion (notably in the elderly)
- loin pain.
It is important to look for these clinical signs of infection before making a diagnosis based on the result of a specimen alone.







