Chapter 8: Nutrition and fluid balance
Skip chapter table of contents and go to main content
Source: Adapted from Sheppard and Wright ([169]) with permission of Elsevier.
Anatomy and physiology
Body composition
The human body is made up of approximately 60% water but this varies with age, gender and percentage of fatty tissue. The higher percentage of fat in women's bodies means a lower water content (approximately 50%), while a higher muscle mass contains a higher water content (Marieb and Hoehn [96], Tortora and Derrickson [183]). Bodily water/fluid is essential to life and vital for:
- controlling body temperature
- the delivery of nutrients and gases to cells
- the removal of waste
- acid‐base balance
- the maintenance of cellular shape (Baumberger‐Henry [20]).
Total body water is distributed between two main compartments: intracellular fluid (ICF, within the cell) and extracellular fluid (ECF, outside the cell) (Table 8.1). ECF is further divided into the intravascular space, within the blood vessels (known as plasma); the interstitial space, which surrounds the cells; and the transcellular space. The transcellular space contains specialized fluids, such as cerebrospinal fluid, that are not readily exchanged with other compartments and so are rarely considered in fluid balance or management (Rhoda et al. [151]).
Table 8.1 Body fluid compartments
| Volume (L) | Distribution | |||
|---|---|---|---|---|
| Total body water, made up of: | 40 | 60% of bodyweight | ||
| Intracellular fluid (ICF) | 25 | 40% of bodyweight | ||
| Extracellular fluid (ECF), made up of: | 15 | 20% of bodyweight | ||
| Interstitial fluid (IF) | 12 | 80% of ECF | ||
| Plasma | 3 | 20% of ECF |
Body fluid is a composition of water and a variety of dissolved solutes known as electrolytes or non‐electrolytes (Marieb and Hoehn [96]) (Figure 8.1). Non‐electrolytes (such as glucose, lipids, creatinine and urea) are molecules that do not dissociate in solution and have no electrical charge. Electrolytes (such as potassium, sodium, chloride, magnesium and bicarbonate) all dissociate in solution into charged ions that conduct electricity. Concentration of these solutes varies depending on the compartment in which they are contained; for example, ECF has a high sodium content (135–145 mmol/L) and is relatively low in potassium (3.5–4.5 mmol/L) and ICF is the reverse: high in potassium but lower in sodium. The movement and distribution of fluid and solutes between compartments are controlled by the semi‐permeable phospholipid cellular membranes (Figure 8.2) that separate them (Tortora and Derrickson [183]).
Transport and movement of water and solutes
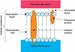
Water can readily and passively pass across the cell semi‐permeable membrane and does so by osmosis (Table 8.2) in response to changing solute concentrations (Tortora and Derrickson [183]). The amount of solute in solution determines the osmolarity – the higher the solute concentration, the higher the osmolarity; this is also referred to as the osmotic pressure (or pull). Electrolytes move across the membrane via the protein channels, some by diffusion (Table 8.2) and some via a passive mode of transport where solutes move towards an area of low solute concentration. Sodium and potassium are exceptions to this rule, as they are required to move against the concentration gradient in order to preserve higher intravascular sodium concentrations. Energy is used to pump sodium out of the cell via the protein channels and pump potassium back into the cell; this is known as the sodium/potassium pump (Figure 8.3).
Table 8.2 Molecule transport modes
| Transport mode | Description | Diagram |
|---|---|---|
| Osmosis | Movement of water from an area of low solute concentration to an area of high solute concentration. | |
| Diffusion | Movement of solutes from an area of high concentration to an area of low concentration. | |
| Facilitated diffusion | Movement of solutes from an area of high concentration to an area of low concentration, facilitated by a carrier molecule (e.g. glucose only enters the cell carried by insulin). | |
| Active transport | Movement of solutes against the concentration gradient from an area of low concentration to an area of high concentration. This mode of transport requires energy synthesized within the cell (i.e. the sodium/potassium pump). |
Table 8.3 Fluid intake and output
| Intake | Output |
|---|---|
| Oral | Urine output |
| Food and drinks | Normally approx. 1500 mL per day |
| Normally 2000 mL per dayc08-note-0001 | Faeces |
| Parenteral/intravenous | Normally approx. 100 mL per day |
| Maintenance fluids, intravenous infusion, intermittent drugs, flushes | Perspiration |
| Additional to or replaces oral intake | Normally approx. 200 mL per day |
| Enteral | Gastric secretions |
| Nasal gastric/nasal jejunostomy, percutaneous gastric jejunostomy feed, flushes | Vomit, nasal gastric/gastrostomy drainage |
| Additional to or replaces oral intake | Additional to normal output |
| Wounds and drains | |
| Additional to normal output | |
| Insensible losses | |
| Perspiration, respiratory secretions | |
| Additional to normal output |
| * | NICE ([122]) recommends a total intake of 30–35 mL/kg; however, this guidance should take into account the patient's clinical condition and careful consideration of their fluid needs at the time. |

Figure 8.3 Active transport: sodium/potassium pump. Source: Clod94, CC BY‐SA 4.0, https://creativecommons.org/licenses/by‐sa/4.0.
The movement of water and solutes out of the intravascular space and into the interstitial space is dependent on opposing osmotic and hydrostatic pressures (Tortora and Derrickson [183]). Hydrostatic pressure is caused by the pumping action of the heart and the diameter (resistance) of the vessels and capillaries; this forces water and molecules that are small enough to pass through the membrane out of the vessel and into the interstitial fluid. Within the vascular system, only the capillaries have semi‐permeable membranes and this is where ‘filtration’ occurs. At the arteriole end of the capillary, the hydrostatic pressure exceeds the osmotic pressure, which results in solutes moving out of the plasma and into the interstitial space. At the venous end, hydrostatic pressure is reduced and the osmotic pressure within the vessel (plasma) is higher so water is pulled back into the vessel and circulating volume (Tortora and Derrickson [183]). The osmotic pressure within the vessels is provided by plasma proteins that are too large to pass through the membrane even under pressure. Conditions such as sepsis or a systemic inflammatory response can cause the membranes to become permeable to plasma proteins; osmotic pressure is then reduced, resulting in an excess of water moving into the interstitial space, which in turn leads to oedema. Pulmonary oedema is caused by this mechanism in the lungs.
Osmolarity and fluid balance
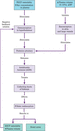
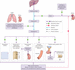
Sodium is the most influential electrolyte in fluid balance and is the primary cation (positively charged ion) of the ECF. The concentration of sodium in the ECF has the most profound effect on its osmolarity and therefore water balance (Rhoda et al. [151]). If ECF osmolarity increases (e.g. with increased intake of sodium, reduced fluid intake or increased fluid loss), even very slightly (by 1–2%), osmoreceptors in the hypothalamus detect and trigger the thirst response (Figure 8.4), which in turn encourages oral fluid intake in an attempt to restore the balance.
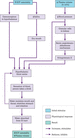
Hormonal mechanisms and the kidneys are highly influential in fluid and electrolyte balance and again are also triggered in response to changing osmolarity and/or plasma volumes. Antidiuretic hormone (ADH) is released from the posterior pituitary gland in response to osmoreceptor (in the hypothalamus) stimulation (Figure 8.5) (Tortora and Derrickson [183]). ADH then acts on the tubules and collecting ducts of the kidneys, inhibiting water excretion and encouraging water reabsorption. If plasma osmolarity falls (indicating water excess), these mechanisms are suppressed by a negative feedback loop and the osmoreceptors are no longer stimulated. This in turn inhibits ADH release; the renal tubules no longer conserve water and thirst is reduced, leading to a reduction of oral intake and restoration of balance. ADH is also released in the renin–angiotensin–aldosterone system (Figure 8.6) response to a reduction in blood pressure (detected by baroreceptors in blood vessels).

Figure 8.6 The renin–angiotensin–aldosterone system. Source: Reproduced with permission of Soupvector – Own work, CC BY‐SA 4.0 https://commons.wikimedia.org/w/index.php?curid=66583851.
Aldosterone is a mineralocorticoid secreted by the adrenal cortex in response to increased osmolarity and/or decreased blood pressure (part of the renin–angiotensin–aldosterone system; Figure 8.6). It acts on the renal tubules, initiating the active transport of sodium (and hence water) from the tubules and collecting ducts back into the plasma and circulating volume (Tortora and Derrickson [183]).
These homeostatic mechanisms are very effective in maintaining fluid and electrolyte balance in health and act to compensate for fluid imbalances to ensure effective cellular function. However, these compensatory mechanisms are not sustainable and will eventually fail if ill health or imbalance persists. For example, in continued haemorrhage, the body will compensate by conserving water and constricting blood vessels in an attempt to increase blood pressure and volume. Failure to replace lost fluids, improve volume and thereby increase perfusion eventually leads to cellular and organ dysfunction, which in turn leads to organ failure and possibly death (Goldstein [60]).
Dehydration is a particular concern in ill health as often fluid intake is reduced by poor appetite, being nil by mouth or experiencing nausea, and often coincides with an increased output due to, for example, vomiting, diarrhoea, haemorrhage, drain output or fever. The elderly are at particular risk of dehydration as the effectiveness of the thirst response diminishes with age (Bak and Tsiami [8], Welch [187]). When the osmolarity of the ECF increases, it encourages water out of the cell and into the ECF, which eventually leads to cellular dehydration, impaired metabolism, disturbed cellular shape and impaired cellular function (Tortora and Derrickson [183]).
If the osmolarity of the ECF falls, water moves into the cell; if this continues, it will lead to water toxicity, causing cells to expand and eventually burst. Care should therefore be taken when administering intravenous fluids (Powell‐Tuck et al. [146], Rhoda et al. [151]), as fluids that are of lower osmolarity than ECF (hypotonic) will cause a shift of water into the cells. Conversely, hypertonic solutions will cause a shift of fluid from the cells, causing cellular dehydration. Maintenance fluids are usually isotonic (have the same osmolarity as ECF) (Gross et al. [63], Rhoda et al. [151]).