Chapter 10: Pain assessment and management
Skip chapter table of contents and go to main content
Source: Adapted from Elliott ([53]), Stewart ([173]).
Pain management
Evidence‐based approaches
Pain management uses a multidisciplinary approach that matches therapy to the individual patient. In some instances, simple analgesia can be sufficient to control pain. Simple or non‐opioid analgesics include paracetamol and non‐steroidal anti‐inflammatory drugs (NSAIDs), used either individually or in combination. Other incidences may require a multimodal approach.
Multimodal analgesia
Multimodal analgesia is the administration of two or more drugs that act by different mechanisms (via the same or different routes) to provide analgesia. This allows for lower doses of individual drugs. It combines different analgesics that act by different mechanisms and at different sites in the nervous system. The aim is to achieve greater analgesia than each of the individual drugs could provide alone and to reduce the patient's opioid requirements and any related side effects (Polomano et al. [141], Rosero and Goshi [161]).
Opioids, non‐opioids (such as paracetamol, NSAIDs, and cyclooxygenase‐2‐selective inhibitors (COX‐2)), local anaesthetics and anticonvulsants are all examples of drugs that may be used as part of a multimodal analgesic approach. An example of multimodal analgesia to manage acute post‐operative pain would be a continuous epidural infusion of a combined opioid and local anaesthetic solution along with paracetamol and an NSAID (if not contraindicated). Another example would be a continuous peripheral nerve block with paracetamol and a NSAID. Both of these approaches combine different analgesic compounds and analgesic approaches (oral route, epidural route and peripheral nerve block).
A multimodal approach may also include non‐pharmacological approaches, such as relaxation therapy, guided imagery, transcutaneous electrical nerve stimulation (TENS) and heat therapy. During the process of devising a multimodal analgesic plan, it is good practice for the prescriber to be aware of any Medicines and Healthcare products Regulatory Agency alerts that may influence their prescribing and management strategy (see https://www.gov.uk/government/organisations/medicines‐and‐healthcare‐products‐regulatory‐agency).
Management of persistent chronic and cancer pain
It has been suggested that despite progress in pain management, chronic non‐cancer pain still represents a clinical challenge, with data demonstrating that chronic pain affects between one‐third and one‐half of the population of the UK – a figure that is likely to increase further, with time, in line with the ageing of the population (Fayaz et al. [56]). Chronic pain has negative impacts on quality of life, interferes with work and causes increased mortality (Moore et al. [117]).
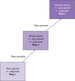
The management of pain is directed by the ‘analgesic ladder’ (also known as the ‘pain ladder’), which was published by the World Health Organization (WHO) in 1986 as a guide to the management of persistent cancer pain (Figure 10.6). Although the analgesic ladder was originally published for the management of cancer pain, it is now widely used by medical professionals for the management of all types of pain, including chronic persistent non‐cancer pain (Balding [6]). It involves a stepwise approach to the use of analgesics, including non‐opioids (step 1), opioids for mild to moderate pain (step 2) and opioids for moderate to severe pain (step 3). Adjuvant drugs are those that contribute to pain relief but are not primarily indicated for pain management. They can be used at all steps of the ladder. Examples include antidepressant and anticonvulsant drugs, corticosteroids, benzodiazepines, antispasmodics and bisphosphonates.
The WHO treatment guide recommends the following five points for the correct use of analgesics (WHO [195]):
- They should be administered orally if appropriate.
- They should be given at regular intervals.
- They should be prescribed according to an assessment of pain intensity evaluated using a pain intensity scale.
- The dose of the analgesic should be adapted to the individual. There is no standard dose to treat certain types of pain.
- Analgesia should be prescribed with ongoing review, monitoring for effectiveness and side‐effects.
Some patients who present with severe pain will need to start on step 3 of the ladder; it is frequently not appropriate for patients to progress through each step in these circumstance. Raffa and Pergolizzi ([146]) discuss how adaptations could potentially be made to the WHO analgesia ladder due to advancements that have been made in the basic science around pain anatomy and physiology, advancements in the breadth of therapeutic treatments available, and a societal shift that has occurred in attitudes toward pain management. They note, however, that it is still a useful construct in devising a pain management strategy, as it provides a series of review points in a clinician's ongoing assessment, although they caution that it should not act as a substitute for evidence‐based guidelines. Carlson ([34]) supported the use of the WHO analgesics ladder, indicating that at least 20% and potentially 100% of patients with cancer pain can be provided with adequate pain relief across the span of their illness, via the application of the WHO's guidelines.
It is important to remember that each patient will experience different types of pain, and individual patients may experience various types of pain, due to aetiological and physiological differences. Each pain needs to be assessed separately, since an individual's pain may need to be managed in various ways and one analgesic intervention or route will rarely be sufficient. Often the best practice is to combine different types of analgesia in order to achieve maximum pain control (Table 10.2). It is also important to use non‐pharmacological interventions at all stages of the treatment plan. And, finally, accurate ongoing assessment is imperative for efficient and effective pain control.
Table 10.2 The use of adjuvant drugs (co‐analgesics)
| Type | Use | Examples |
|---|---|---|
| Non‐steroidal anti‐inflammatory drugs (NSAIDs) |
Bone pain
Muscular pain
Inflammation
Visceral pain |
Diclofenac
Naproxen
Ibuprofen |
| Steroids |
Pressure
Bone pain
Inflammation
Raised intracranial pressure |
Dexamethasone
Prednisolone |
| Tricyclic antidepressants | Neuropathic pain | Amitriptyline |
| Anticonvulsants | Neuropathic pain |
Sodium valproate
Carbamazepine
Gabapentin
Pregabalin |
| Antibiotics | Infection |
Flucloxacillin
Trimethoprim |
| Benzodiazepines | Anxiety |
Diazepam
Clonazepam |
| Antispasmodics | Spasms | Baclofen |
| Bisphosphonates | Bone pain |
Sodium clodronate
Disodium pamidronate
Zoledronic acid |
Using the WHO analgesic ladder
The analgesic ladder (see Figure 10.6) was designed as a framework for the pharmacological management of cancer pain and is also often used to manage chronic pain. There are several pharmacological agents available to manage pain and the analgesic ladder allows the flexibility to choose from this range according to the patient's requirements and tolerance. In 2010, new guidance on the analgesic ladder promoted its bidirectional use with a ‘step up, step down’ approach, proposing an upward pathway for the treatment of cancer pain and a downward pathway for the treatment of intense acute pain, uncontrolled chronic pain and breakthrough pain (Vargas‐Schaffer [186]).
For acute pain management, the WHO ladder can be used as a guide in reverse, starting at step 3 for immediate post‐operative pain and moving down through step 2 and then step 1 as post‐operative pain improves.
The principles of the WHO analgesic ladder are to provide a therapeutic effect alongside all other interventions (both pharmacological and non‐pharmacological), to guide the patient's treatment and help in managing their underlying condition, to prevent avoidable complications, and to maintain or improve quality of life (Vargas‐Schaffer and Cogan [187]).
Step 1: non‐opioid drugs
Examples of non‐opioid drugs include paracetamol, aspirin and NSAIDs. NSAIDs are especially effective for pain from inflammation. All of the examples of non‐opioid drugs given in Table 10.2 are used to manage mild to moderate pain and can also be used as adjuncts to opioid analgesia to manage moderate to severe pain (Polomano et al. [141]).
Step 2: opioids for mild to moderate pain
Examples of opioids for mild to moderate pain include codeine, dihydrocodeine, tramadol and low‐dose oxycodone (steps 2 and 3). These drugs are used when non‐opioids are unable to achieve adequate pain control and are usually used in combination formulations. It is not recommended to administer another step 2 analgesic if pain remains uncontrolled. Uncontrolled pain needs to be assessed and managed with the titration of an opioid by moving up the ladder. The exception to this would be if the patient was experiencing intolerable side‐effects on the weak opioid and an alternative drug might be beneficial.
Step 3: opioids for moderate to severe pain
Examples of opioids for moderate to severe pain include morphine, oxycodone, fentanyl, diamorphine, methadone, buprenorphine, hydromorphone and alfentanil.
Methods of drug delivery
Oral analgesia
Oral opioids are used less frequently in the immediate post‐operative period because many patients are nil by mouth or on restricted oral intake for a period of time. Often this route is used if patients require strong analgesics following discontinuation of epidural or intravenous analgesia. Morphine is an ideal oral preparation because it is available as a tablet (Sevredol) or an elixir (Oramorph). Oxycodone can be given as a second‐line opioid treatment if patients are allergic or sensitive to morphine, or fail to respond to it.
Intravenous analgesia
Continuous intravenous infusions of opioids such as morphine, diamorphine and fentanyl are effective for controlling pain in the immediate post‐operative period. Their use is often restricted to critical care units, where patients can be closely monitored, because of the potential risk of respiratory depression (Macintyre and Schug [99]). The need for this restriction is reinforced by Lee et al. ([93]), who found that 88% of respiratory depression events occurred within 24 hours of a surgical procedure. A meta‐analysis found that compared with patient‐controlled analgesia (PCA) using bolus only, the addition of a continuous or background infusion was associated with an increased risk of respiratory depression (George et al. [67]).
Patients may control post‐operative pain via self‐administration of intravenous opioids using devices designed for this purpose (i.e. PCA). In a Cochrane review, McNicol et al. ([110]) found evidence that PCA is an efficacious alternative to non‐patient‐controlled systemic analgesia for post‐operative pain control, with patients demonstrating lower visual analogue scale pain intensity scores versus patients not using PCA over most time intervals. When in pain, the patient presses a button connected to the pump and a set dose of opioid is delivered (usually intravenously but it may also be given subcutaneously or via epidural) (Macintyre and Schug [99]).
There are a number of advantages to using PCA:
- PCA is more likely than non‐patient‐controlled systemic analgesia to maintain reasonably constant blood concentrations of the drug within the analgesic corridor (the blood level where analgesia is achieved without significant side‐effects). The flexibility of PCA helps to overcome the wide inter‐patient variation in opioid requirements (Macintyre and Schug [99]).
- PCA allows for intra‐patient variability in analgesic needs, allowing patients to titrate analgesia according to daily variations in their pain stimulus (Hurley et al. [83]). By using a PCA pump, patients can administer analgesia as soon as pain occurs and titrate the dose according to increases and decreases in the pain stimulus. This is particularly helpful for controlling more intense pain during movement or other activities that can trigger pain.
- PCA gives patients better autonomy and control over the amount of medication used (Garimella and Cellini [64]).
While PCA may be very effective for controlling pain for a number of patients, particularly those who have undergone surgery (Macintyre and Schug [99]), it is not suitable for the groups listed in Box 10.3.
Box 10.3
Patients unsuitable for PCA
- Those with poor manual dexterity or severe visual impairment, who may be physically incapable of activating the demand button
- Those with cognitive impairment (such as confusion) or learning difficulties who are unable to understand the concept of PCA and how to use the device
Epidural analgesia
Epidural analgesia refers to the provision of pain relief by continuous administration of analgesic pharmacological agents (usually low concentrations of local anaesthetics and opioids) into the epidural space via an indwelling catheter (Schug et al. [166]). Giving analgesia epidurally is a particularly valuable technique for the prevention of post‐operative pain in patients undergoing major thoracic, abdominal or lower limb surgery, and can sometimes be used to manage the pain associated with trauma. Commonly used opioids for epidural analgesia include fentanyl and morphine (Mariano [104]). Combinations of low concentrations of local anaesthetic agents and opioids have been shown to provide consistently superior pain relief compared with either drug alone (Macintyre and Schug [99]).
Subcutaneous analgesia
Opioids are often given subcutaneously to manage chronic cancer pain. More recently, there has been an increase in the use of subcutaneous opioids for post‐operative pain control. Both PCA and nurse‐administered opioid injections of morphine, diamorphine or oxycodone via an indwelling subcutaneous cannula have been used successfully to manage post‐operative pain (Macintyre and Schug [99]). An advantage of giving analgesia subcutaneously is that it avoids the problems associated with maintaining intravenous access.
Intramuscular analgesia
Until the early 1990s, regular (every 3–4 hours) intramuscular injections of opioids, such as pethidine and morphine, were routinely used for the management of post‐operative pain. Because alternative techniques, such as PCA and epidural analgesia, are now available, intramuscular analgesia is used less frequently. Some useful algorithms have been developed to give guidance on titrating intramuscular analgesia (Harmer and Davies [73], Macintyre and Schug [99]). Absorption via this route may be impaired in conditions of poor perfusion (e.g. in hypovolaemia, shock, hypothermia or immobility). This may lead to inadequate early analgesia (where the drug cannot be absorbed properly and reach the systemic circulation and so forms a drug depot) and late absorption of the drug depot (where the drug has remained in the muscular tissue and is absorbed only once perfusion is restored) (Schug et al. [166]).
Transdermal analgesia
Transdermal analgesia is a simple method of giving analgesia. It is convenient and often very acceptable to patients, particularly those who dislike tablets or have many to take. A number of patch formulations have been developed to allow the delivery of drugs across the skin (such as fentanyl, buprenorphine or local anaesthetics). Disadvantages of giving strong opioids such as fentanyl by this route include inflexibility (the patient usually has to be on a stable dose of an opioid and it takes a long time for a dose increase to take effect), and breakthrough doses must be given by another route (oral, buccal or sublingual). Transdermal fentanyl (except for iontophoretic patient‐controlled transdermal devices) should not be used in the management of acute pain because of safety concerns and difficulties in the short‐term dose adjustments needed for titration (Schug et al. [166]).
Buccal and sublingual analgesia
Buccal means the analgesia is placed between the upper lip and the lining of the upper gum. A sublingual drug is placed under the tongue. Drugs given by this route pass directly into the systemic circulation and bypass first‐pass hepatic metabolism. Their speed of onset is often rapid as this method avoids the harsh acidic environment of the stomach and the enzymatic milieu of the small intestine (Sattar et al. [164]).