Chapter 12: Respiratory care, CPR and blood transfusion
Skip chapter table of contents and go to main content




Source: Adapted from Estcourt et al. ([74]) with permission of John Wiley & Sons.
Evidence‐based approaches
The transfusion of blood components is a complex multistep process involving personnel from various professional backgrounds with differing levels of knowledge and understanding. Errors made in the process of transfusion present a significant risk to patients, and SHOT highlights that a team approach with good communication is essential (Narayan [172]). Every step should have the necessary checks carried out in full to ensure any errors in the steps prior are identified.
The evidence to support the efficacy of set procedures to manage this risk has been evaluated and provided in recommendations by the British Society for Haematology (BSH) and the SHOT reports (Bolton‐Maggs [27], Robinson et al. [236]). As a result of this guidance, every hospital should have a policy for the provision and administration of blood components that includes all steps of the process outlined in Figure 12.73. Furthermore, hospitals are required to manage and report any adverse events or near misses, and there is a statutory requirement to hold a record of every step of the transfusion process, including the final fate of each blood product, for 30 years (BSQR [34], JPAC [121]). Only authorized staff should be involved at any stage in the transfusion process.

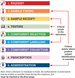
Figure 12.73 Vital steps involved in the transfusion of blood components.
Source: Reproduced from Bolton‐Maggs ([27]) with permission of Serious Hazards of Transfusion.
In the UK, nurses are usually the healthcare professionals ultimately responsible for the bedside check that is carried out before administering the blood component. The bedside check is the final opportunity to prevent patients from receiving the incorrect component, including recognizing any missing specific requirements (Bolton‐Maggs [27]). Errors in the requesting, collection and administration of blood components have led to significant harm and sometimes fatal consequences. Since its launch in 1996, the SHOT scheme has continually shown that ‘wrong blood into patient’ episodes are a frequently reported transfusion hazard. These ‘wrong blood’ incidents are mainly caused by human error arising from misidentification of the patient during blood sampling, errors in blood component collection and delivery, and errors in administration; these can lead to life‐threatening haemolytic transfusion reactions and other significant morbidity (Bolton‐Maggs [27]). In the 2017 SHOT report, the cumulative data submitted since 1996 was analysed to assess the likelihood of preventability (Bolton‐Maggs [27]). It was found that half of all reported incidents had been preventable, adverse events caused by mistakes, such as assuming patient identity or not following the correct checking processes (Figure 12.74). Furthermore, the National Comparative Audit (NCA) of bedside transfusion practice shows that patients are placed at risk of avoidable complications of transfusion through misidentification and inadequate monitoring (NHSBT [184], [185], [186]).

Figure 12.74 Cumulative data for SHOT categories 1996 to 2017, n = 19,815.
Source: Adapted from Bolton‐Maggs ([27]) with permission of Serious Hazards of Transfusion.
Due to the continuing risk of ‘wrong blood’ incidents, despite interventions such as competency assessments for transfusion, a Central Alerting System (DH [64]) has been issued mandating the use of a bedside checklist for transfusion administration (Figure 12.75).

Figure 12.75 Bedside checklist.
Source: Reproduced from Bolton‐Maggs ([27]) with permission of Serious Hazards of Transfusion.
Rationale
Blood transfusion is an essential part of modern medicine and potentially a life‐saving intervention. However, the use of blood components should be appropriate and limited when possible, and alternative treatments – such as pharmacological interventions, and good surgical and anaesthetic techniques – should be considered (AAGBI [1], NBTC [176], NICE [191], WHO [283]). The National Blood Transfusion Committee for England describes patient blood management as evidence based and multidisciplinary, encompassing measures to reduce the need for transfusion (NBTC [176]). These recommendations were further echoed by NICE ([191]), which advocates measures to decrease the need for transfusion during surgery and to restrict the use of transfusion in all patients. Only when options to minimize the need for a transfusion have been implemented should a transfusion be considered. The pillars of patient blood management can be seen in Figure 12.76.

Figure 12.76 The pillars of patient blood management.
Source: Reproduced from Shander et al. ([252]) with permission of John Wiley & Sons.
Recent publications and recommendations (Narayan [172], NBTC [176], NICE [191]) have created greater awareness of the need to continue to improve transfusion practice in many ways. Blood components are no longer regarded as safe, unlimited resources. There are risks inherent in transfusion practice and therefore unnecessary exposure to blood components should be avoided. This is of particular importance for patients who may only have one transfusion in their lifetime, such as surgical patients. However, all patients should only receive a transfusion when it is absolutely necessary. Furthermore, the appropriate use of blood and its components is essential for the conservation of blood supplies. Regularly updated guidance on the safe and appropriate use of blood and blood components is available online at www.transfusionguidelines.org.uk and this should be consulted in collaboration with local hospital and national blood transfusion guidelines.
Red cells: indications
The WHO defines anaemia and its severity by the haemoglobin concentration in blood (Pasricha et al. [213]). A person is considered anaemic when their haemoglobin level is below the expected value, taking into consideration age, biological sex and pregnancy. In general, anaemia is a consequence of one or more of the following generic causes:
- increased loss of red blood cells
- decreased production of red blood cells
- increased destruction of red blood cells
- increased demand for red blood cells
- increased production of abnormal red blood cells.
The cause of anaemia should be ascertained and possible effective treatment options explored, such as treatment of iron deficiency before the patient is transfused with red cells (NBTC [176]). Pasricha et al. ([213]) highlight that when choosing a treatment for anaemia it is important to take into consideration the severity of the patient's symptoms and the clinical effects of the anaemia as well as its chronicity.
Due to the variability of patient co‐morbidities and anaemia tolerance, there is little evidence to support a generic ‘transfusion trigger’ (a haemoglobin level that requires subsequent blood transfusion). Evidence suggests that most patients can safely tolerate anaemia of 70 g/L of haemoglobin in the absence of active bleeding (NICE [191]). A systematic review exploring the evidence on haemoglobin thresholds for blood transfusion concluded that restrictive transfusion triggers were favourable in the majority of patients and that a haemoglobin threshold of 70 or 80 g/L lowers the number of red cell units transfused (Carson et al. [38]). The review also concluded that restrictive transfusion triggers had no adverse effects on cardiac morbidity, mortality or length of hospital stay (Carson et al. [38]).
Red cells: contraindications
NICE ([191]) recommends that patients without acute coronary syndrome should have a restrictive transfusion approach and a post‐transfusion target threshold of 70–90 g/L, and that those with acute coronary syndrome should have a post‐transfusion threshold of 80–100 g/L. Patients who have cardiovascular disease, renal disease, low albumin concentration or are of low bodyweight (in particular, the elderly and children) may also be more susceptible to volume overload leading to congestive cardiac failure when blood and other fluids are infused (McClelland [151], Norfolk [202]). SHOT (Bolton‐Maggs [27], Narayan [172]) has developed a checklist for risk factors for transfusion‐associated circulatory overload (TACO) (Figure 12.77). In 2017, there were seven patient deaths reported to SHOT that were attributed to over‐transfusion and TACO. The 2017 SHOT report (Bolton‐Maggs [27]) and the BSH (Robinson et al. [236]) recommend a formal risk assessment for TACO prior to each transfusion. NICE ([191]) and the BSH (Robinson et al. [236]) recommend that, to help mitigate the risk of TACO, single‐unit transfusions should be given in stable, non‐bleeding patients and then further assessment of the patient should be made prior to administering further units.

Figure 12.77 Transfusion‐associated circulatory overload (TACO) safety checklist.
Source: Reproduced from Bolton‐Maggs ([27]) with permission of Serious Hazards of Transfusion.
Red cell transfusions are contraindicated when the underlying cause of the anaemia has a non‐transfusion treatment available. For example, iron deficiency anaemia should be treated with oral or intravenous iron infusion (NICE [196]), or B12 treatment in the case of pernicious anaemia. Red cell transfusions are also limited in patients who are potential renal transplant recipients (Norfolk [202]) to limit the risk of alloimmunization and subsequent transplant rejection.
Platelets: indications
Platelet transfusions are indicated in the prevention and treatment of haemorrhage in patients with thrombocytopenia or platelet function defects (Estcourt et al. [74]). Thrombocytopenia can be defined as a platelet count below the normal range for the population, which is usually considered to be between 150 and 450 × 109/L (Weil et al. [282]). Thrombocytopenia is usually caused by either decreased platelet production or increased destruction. Not all thrombocytopenic patients require a platelet transfusion and in some cases it may be contraindicated. Patients who have chronic stable thrombocytopenia due to such conditions as myelodysplasia and aplastic anaemia often do not require a transfusion and do not experience haemorrhage even with platelet counts below 10 × 109/L (Estcourt et al. [74]). In prophylactic situations there are various thresholds, depending on the reason for the transfusion and the presence of risk factors for bleeding; see Table 12.22 for adult thresholds for transfusion.
Table 12.22 Indications for the use of platelet transfusions in adults
| Indication | Transfusion indicated (threshold)/not indicated |
|---|---|
| Prophylactic use (no bleeding or WHO grade 1) | |
| One adult dose required | |
| Reversible BMF including allogeneic stem cell transplantation | 10 × 109/L |
| Reversible BMF with autologous stem cell transplantation (consider no prophylaxis) | 10 × 109/L |
| Critical illness | 10 × 109/L |
| Chronic BMF receiving intensive therapy | 10 × 109/L |
| Chronic BMF to prevent persistent bleeding of grade ≥2 | Count variable |
| Chronic stable BMF, abnormal platelet function, platelet consumption/destruction (e.g. DIC, TTP) or immune thrombocytopenia (HIT, ITP, PTP) | Not indicated |
| Prophylactic use in the presence of risk factors for bleeding (e.g. sepsis, antibiotic treatment, abnormalities of haemostasis) | |
| Reversible/chronic bone marrow failure/critical care | 10–20 × 109/L |
| Abnormal platelet function, platelet consumption/destruction, immune thrombocytopenia | Not indicated |
| Platelet transfusion pre‐procedure | |
| CVC excluding PICC line | 20 × 109/L |
| Lumbar puncture | 40 × 109/L |
| Percutaneous liver biopsy | 50 × 109/L |
| Major surgery | 50 × 109/L |
| Epidural anaesthesia, insertion and removal | 80 × 109/L |
| Neurosurgery or ophthalmic surgery involving the posterior segment of the eye | 100 × 109/L |
| Bone marrow aspirate or trephine biopsies, PICC line insertion, traction removal of CVCs, cataract surgery | Not indicated |
| Therapeutic use (bleeding WHO grade 2 or above) | |
| Severe bleeding | 50 × 109/L |
| Multiple trauma, brain or eye injury, spontaneous intracerebral haemorrhage | 100 × 109/L |
| Bleeding (WHO grade ≥2) but not severe | 30 × 109/L |
| Bleeding in specific clinical conditions – see below for indications | |
| Specific clinical conditions | |
| Platelet function defect | Count variable |
| Congenital: pre‐procedure or therapeutic use; when alternative therapy contraindicated or ineffective; directed by specialist in haemostasis | |
| Acquired (anti‐platelet agents, uraemia): only indicated for severe bleeding | |
| Disseminated intravascular bleeding: pre‐procedure or therapeutic use; consider threshold counts above but may not be achievable and individual case review required | Use pre‐procedure/therapeutic threshold as guide |
| Thrombotic thrombocytopenic purpura: platelet transfusion contraindicated unless life‐threatening bleeding | Count variable |
| Immune thrombocytopenia (ITP, HIT, PTP): pre‐procedure when other therapy ineffective or procedure urgent, or to treat severe bleeding; consider threshold counts above but may be unachievable or unnecessary and individual case review required | Use pre‐procedure/therapeutic threshold as guide |
| BMF, bone marrow failure; CVC, central venous catheter; DIC, disseminated intravascular coagulation; HIT, heparin‐induced thrombocytopenia; ITP, primary immune thrombocytopenia; PICC, peripherally inserted central catheter; PTP, post‐transfusion purpura; TTP, thrombotic thrombocytopenic purpura; WHO, World Health Organization. See Estcourt et al. ([74]) for an explanation of the World Health Organization's bleeding scale. |
In all cases of prophylactic platelet transfusions, the patient should receive one adult therapeutic dose (one pool or pack) and then be reassessed. Giving double‐dose platelet transfusions does not decrease the risk of the patient bleeding (Slichter et al. [260]). There should be careful assessment of the need for a platelet transfusion as the effectiveness and the platelet rise following the transfusion will start to reduce as the number of platelet transfusions increases (Slichter et al. [260]). Following the transfusion, the platelets’ effectiveness should be evaluated by checking the increment; a sample should be taken 10 minutes after completion of the transfusion. One adult dose of platelets in a 70 kg adult typically gives a rise of 20–40 × 109/L (NHSBT [187], O'Connell et al. [207]).
Platelets: contraindications
Platelets are contraindicated in patients with microangiopathies such as thrombotic thrombocytopenic purpura and should only be used to treat life‐threatening bleeding, as recommended in the BSH's 2017 guidelines (Estcourt et al. [74]). Prophylactic platelets are also not advised for patients with immune thrombocytopenia (Estcourt et al. [74]). Platelet transfusions should only be used in severe bleeding for patients with post‐transfusion purpura (Eldin and Teruya [71]).
Fresh frozen plasma (FFP) and pathogen‐inactivated FFP: indications
Plasma is mainly used for patients who are bleeding due to multiple clotting factor deficiencies such as disseminated intravascular coagulation or to replace clotting factors following massive haemorrhage (Hunt et al. [114], Norfolk [202]). The BSH guidelines report that the evidence base to support the use of plasma to correct clotting in non‐bleeding patients is lacking (Green et al. [94]); however, plasma is often given prior to invasive procedures to prevent bleeding, and abnormal clotting results do not predict bleeding risk (Green et al. [94]). In major haemorrhage episodes, FFP is recommended by the BSH to be given at a minimum ratio of 1 FFP to 2 red cells until coagulation results are available (Hunt et al. [114]).
Patients born after 1 January 1996 require imported and pathogen‐inactivated plasma; this is to reduce the risk of infectious agents. The date is significant in that acquisition of vCJD (variant Creutzfeldt–Jakob disease) via diet is ‘assumed to have ceased’ by this time (Norfolk [202], p.59). The component is imported from countries at low risk for vCJD.
Fresh frozen plasma (FFP) and pathogen inactivated FFP: contraindications
FFP is not indicated for warfarin reversal (Norfolk [202]). It is also not indicated in cases of hypervolaemia to support circulating volume (Green et al. [94]).
Further guidance on the use (in various patient groups) and handling of FFP and cryoprecipitate products can be found in the BSH guidelines (Green et al. [94]).
Cryoprecipitate and pathogen‐inactivated cryoprecipitate: indications
The main constituents of cryoprecipitate are fibrinogen, factor VIII and von Willebrand factor (Norfolk [202]). It is clinically indicated in major haemorrhage when fibrinogen supplementation is required (Hunt et al. [114]). The BSH guidelines highlight that there is limited evidence for a threshold or level when cryoprecipitate would be beneficial, but a fibrinogen level of below 1.5 g/L in a bleeding patient is given to guide clinical practice (Hunt et al. [114]). In non‐bleeding situations, such as an invasive procedure, there again is a lack of evidence to support recommendations. The recommendation is to assess patients’ risk of bleeding by undertaking an assessment of previous bleeding episodes or any familial history of bleeding and, when a significant risk is identified, to consider transfusion when the fibrinogen level is below 1.0 g/L (Green et al. [94]).
Patients born after 1 January 1996 require imported and pathogen‐inactivated cryoprecipitate for the same reason as they require imported and pathogen‐inactivated plasma (see above).
Cryoprecipitate and pathogen‐inactivated cryoprecipitate: contraindications
Cryoprecipitate should not be used to treat clotting factor deficiencies when a clotting factor concentrate or pharmaceutical agent is available. For example, it should not be used to treat factor VIII deficiency in haemophilia, to treat von Willebrand factor deficiency, or for warfarin reversal (Eldin and Teruya [71]).
Methods
Blood donation and testing
All blood donated in the UK is given voluntarily and without remuneration. The successful selection of a donor involves protecting them from any harm that could be caused by the donation process and also protecting the possible recipient of components derived from the donor's blood. Donors of blood for therapeutic use should be in good health; if there is any doubt about their suitability, the donation should be deferred and they should be fully assessed by a designated medical officer. All donors of blood or its components (via apheresis) should be assessed in accordance with the Joint UK Blood Transfusion Society and National Institute for Biological Standards and Control Professional Advisory Committee (JPAC) donor selection guidelines (JPAC [121]). The assessment of fitness to donate includes a questionnaire relating to general health, lifestyle, past medical history and medication. Donation may be temporarily or permanently deferred for a variety of reasons, including cardiovascular disease, central nervous system diseases, malignancy and some infectious diseases, all of which are detailed in the JPAC ([121]) guidelines. Donors are also screened for risk of exposure to transmissible infectious diseases, and specific guidance is provided for donors receiving therapeutic drugs.
Prevention of the transmission of infection is determined by donor selection criteria and laboratory testing. In the UK, all blood donations are tested for infections that could be passed on to the recipient. Transfusion‐related vCJD transmission has been reported in four cases (Seed et al. [247]). Although in 2011 a prototype blood test for diagnosis of vCJD in symptomatic individuals was developed by the Medical Research Council's Prion Unit, there is still no available high‐throughput and specific screening test (Seed et al. [247]). At present, donor exclusion criteria and leucodepletion remain as precautionary measures for all infection including vCJD (Seed et al. [247]). Since April 2004, all individuals who have received a blood component since January 1980 have been excluded from donating blood due to the risk of transmitting vCJD (DH [63]). When a donor has been successfully screened, they must validate the information they have provided and record that they have given consent to proceed.
Cell salvage and autologous transfusion
Since the 1980s, there has been interest in autologous transfusion (blood collected from an individual and intended solely for subsequent autologous transfusion to that same individual) (BSQR [34]). The objective of autologous transfusion is to decrease the need for allogeneic blood transfusion and the associated risks and costs (Sikorski et al. [256]). Autologous transfusion is used primarily for surgical patients, as explained below. It is not risk free, and SHOT reports the adverse reactions and events for the UK. In 2017, 17 cases were reported in relation to cell salvage; however, there was no major morbidity (Bolton‐Maggs [27]). Autologous transfusion is contraindicated in certain circumstances; however, in cases of surgery with large anticipated blood loss, patients can benefit from the use of cell salvage (Sikorski et al. [256]). There have been concerns over using cell salvage in cancer surgery in case it leads to circulating tumour cells and causes tumour dissemination (Sikorski et al. [256], Zaw et al. [293]). However, it is now known that this does not cause metastases (NICE [189], Zaw et al. [293]). A Cochrane review of pre‐autologous donation in 2002 concluded that it was difficult to say whether the benefit outweighs the harm (Henry et al. [104]) and it is now rarely undertaken by hospitals. Three principal methods of autologous transfusion exist: pre‐operative autologous donation (PAD), acute normovolaemic haemodilution (ANH), and either intraoperative cell salvage (ICS) or post‐operative cell salvage.
Pre‐operative autologous donation
PAD is rarely undertaken and not currently recommended unless the clinical circumstances are exceptional – for example, if the patient has a rare blood group and allogeneic blood would be difficult to obtain (BCSH et al. [17]). It requires the patient to donate up to four units of blood while simultaneously taking iron supplements in the month preceding surgery. This technique can only be carried out in organizations licensed as blood establishments by the Medicines and Healthcare products Regulatory Agency (MHRA) under the Blood Safety and Quality Regulations ([34]).
Acute normovolaemic haemodilution
ANH is not currently encouraged and the effectiveness of the procedure is unproven (Norfolk [202]). It involves the donation of up to three units of blood immediately prior to surgery. The patient is then given crystalloids to dilute the circulating volume. This method is only indicated for surgery where considerable blood loss is expected on the principle that the number of red cells lost will be reduced and the patient's autologous whole blood can be returned after surgery.
Intraoperative cell salvage
In ICS, blood loss during surgery is collected, anticoagulated, filtered and held in a sterile reservoir. The collected blood is then processed, washed and suspended in 0.9% sodium chloride for return. NICE ([191]) recommends the use of cell salvage in conjuction with tranexamic acid for patients who are having surgery likely to result in high blood loss. Cell salvage has been shown to be effective in reducing the requirement for perioperative allogeneic blood transfusion in orthopaedic, cardiac and vascular surgery (Carless et al. [37]). The effectiveness of ICS to minimize a patient's exposure to allogeneic blood is dependent on the amount of blood lost during surgery, and better surgical techniques have resulted in less blood loss during surgery (NICE [191]).
Post‐operative cell salvage
In post‐operative cell salvage, where there is predictable blood loss following elective surgery, the blood is collected in the wound drain and then reinfused to the patient through special equipment. The blood can be passed through a filter incorporated into the cell salvage post‐operative equipment or washed before being returned to the patient. This has become almost routine for some orthopaedic procedures, mainly hip and knee surgery (Norfolk [202]).
Several cases have been reported to SHOT (Bolton‐Maggs [25]) where the autologous blood had not been labelled with the correct patient identification and in some cases this had not been noted by staff in the clinical area prior to reinfusion (Bolton‐Maggs [25]). SHOT highlighted that it is still critical to maintain correct patient identification in autologous transfusion.
In 2006 a UK cell salvage group was founded to support the implementation of cell salvage; more information and advice can be found at JPAC ([122]). The group produces a wide range of materials including training and competency documents, patient factsheets and guidance on the provision of cell salvage.
Blood component donation
Donors of blood components via automated apheresis are subject to the same selection criteria used for donating whole blood and any exception to this must be decided by a designated medical officer. Apheresis can be used to collect plasma, red cells and platelets. Leukapheresis procedures are used for the collection of granulocytes, lymphocytes and peripheral blood progenitor cells (JPAC [121]).
Appropriate use of donated blood components
Donated blood components are not a limitless resource and must be used appropriately. The BSH has guidelines in place for the use of red cell, platelet, FFP, cryoprecipitate and cryosupernatant transfusions (Estcourt et al. [74], Green et al. [94], Hunt et al. [114], Retter et al. [233]). The decision to transfuse must be based on a thorough clinical assessment of the patient and their individual needs. Each blood component should only be given after careful consideration, when there is a valid clinical indication or when there are no alternative treatment options available (NICE [191]).
Blood and blood components have varying shelf lives and storage requirements. The range of components currently available, indications for their use and recommendations for their administration are listed in Table 12.23. Clinical indications for use are also provided by the BSH (Estcourt et al. [74], Green et al. [94], Hunt et al. [114], Retter et al. [233]).
Table 12.23 Blood, blood components and blood products used for transfusion
| Type | Description | Indications | Cross‐matching | Shelf life | Average infusion time | Technique | Special considerations |
|---|---|---|---|---|---|---|---|
| Red cells in optimal additive solutions (SAGM)c12-note-0008 | Red cells with plasma removed: 100 mL additive fluid used as replacement to give optimal red cell preservation; haematocrit 60–65% leuco‐depleted | Correction of anaemia | ABO and Rh compatible (not necessarily identical) | 35 days at 2–6°C |
1.5–2 hours/unit
Transfusion to be completed within 4 hours of component's removal from storage
Those patients at risk of TACO should have careful monitoring | Give via a blood administration set | If more than half blood volume is replaced with red cells in SAGM, use of FFP should be considered to replace clotting factors |
| Washed red blood cells |
Red cells centrifuged and resuspended twice in 0.9% sodium chloride
Leuco‐depleted | Correction of anaemia where patient may react to plasma components, for example in IgA deficiency when the patient has formed an anti‐IgA | As above |
Prepared by non‐sterile process, used within 24 hours
Closed system preparation, used within 14 days | As above | As above | – |
| Frozen red blood cells |
Red cells of very rare phenotype
Leuco‐depleted | To treat patients with very rare antibodies | As above |
Stored frozen cells: up to 10 years
Use within 24 to 72 hours depending on preparation | 2–3 hours/unit | As above | – |
| White blood cells: granulocytes | Pooled granulocytes obtained by pooling the white cells from whole blood donations | To treat patients with life‐threatening granulocytopenia | As above | Until midnight 1 day after donation | 60–90 minutes/unit | Administer via a blood administration set |
White blood cell infusion induces fever and may cause hypotension, rigors and confusion
Treat symptoms and reassure patient
White cell component is always irradiated to prevent initiation of TA‐GVHD
Do not give to patients receiving amphotericin B
For the clinical indications and contraindications for granulocyte transfusions, refer to the NHS Blood and Transplant guidelines (Massey, [149]) |
| Plateletsc12-note-0008 |
Platelets in 30–35% plasma and 65–70% platelet additive solution
May be pooled from four whole blood donations or apheresed from a single donor
Leuco‐depleted | To treat thrombocytopenia either for prophylaxis to prevent bleeding or therapeutically to treat bleeding | No cross‐matching necessary |
Up to 7 days after collection
Storage is at 22°C with continuous gentle agitation | 30–60 minutes/unit |
Administer using a platelet or blood component administration set
Use a new set for each transfusion
Do not use micro‐aggregate filters |
General guide to use: use in chronic bone marrow failure for routine prophylaxis is not indicated
Platelets are not clinically indicated for a bone marrow aspirate and trephine regardless of the cause of thrombocytopenia
See also Table 12.22
|
| Fresh frozen plasma | Citrated plasma separated from whole blood |
To treat multifactor deficiencies associated with severe bleeding and/or DIC
FFP is not indicated in DIC without bleeding or for the immediate reversal of warfarin | No cross‐matching necessary |
3 years at <−25°C
Once thawed, kept at 4°C and used as soon as possible but within 24 hours | 10–20 mg/kg (approx. 250 mL); more rapid infusion may be indicated in major haemorrhage | Administer rapidly via a blood administration set |
FFP should be considered if the patient has received more than half their blood volume in red cells, to prevent dilutional hypocoagulability
The dose given is based on the patient's weight |
| Albumin 4.5% (HAS) |
Solution of albumin from pooled plasma in a buffered, stabilized 0.9% sodium chloride diluent
Supplied in 250 mL or 500 mL bottle |
To treat hypovolaemic shock or hypoproteinaemia due to burns, trauma, surgery or infection
Sourced outside the UK to reduce the risk of transmission of vCJD | No cross‐matching necessary |
5 years at room temperature
Kept in the dark | 30–60 minutes/unit | Administer via a standard solution administration set | The solution should be crystal clear with no deposits |
| Albumin 20% (HAS) | Heat‐treated, aqueous, chemically processed fraction of pooled plasma |
To treat hypovolaemic shock or hypoproteinaemia due to burns, trauma, surgery or infection
To maintain appropriate electrolyte balance | No cross‐matching necessary |
5 years at room temperature
Kept in the dark | 30–60 minutes/unit |
Administer via a blood administration set undiluted or diluted with 0.9% sodium chloride or 5% glucose solution
Slower administration is advised if a cardiac disorder is present to avoid gross fluid shift | The solution should be crystal clear with no deposits |
| Cryoprecipitate | Cold‐insoluble portion of plasma recovered from FFP: rich in factor VIII, von Willebrand factor and fibrinogen |
To treat hypofibrinogenaemia, in acute DIC with bleeding and surgery prophylaxis with fibrinogen <1.5 g/L
To treat severe liver disease with bleeding and in cases of massive transfusion | No cross‐matching necessary |
3 years at <−25°C
Use immediately after thawing | Available as single donor units or as pooled units (five single‐donor units); typical adult dose is two pooled packs, administered at 10–20 mL/kg/hr, 30–60 minutes as a pooled unit | Administer via a blood administration set | – |
|
Solvent detergent treated FFP
Licensed medicinal product Octaplas | FFP prepared from pools of donations; the solvent detergent process inactivates bacteria and most encapsulated viruses | Guidelines recommend use in treating thrombotic thrombocytopenic purpura: patients are plasma‐exchanged daily to reduce circulating von Willebrand factor | No cross‐matching necessary |
4 years at <−18°C
Use immediately after thawing | Time depends on machine; average approximately 2.5 hours | Via apheresis machine | – |
| DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; HAS, human albumin solution; IgA, immunoglobin A; SAGM, saline, adenine, glucose and mannitol; TACO, transfusion‐associated circulatory overload; TA‐GVHD, transfusion‐associated graft‐versus‐host disease; vCJD, variant Creutzfeldt–Jakob disease. | |
| a | Most commonly used blood components. |
Anticipated patient outcomes
Blood component transfusion can be life saving; however, the focus is now on optimizing patients’ own blood and trying to limit the need for transfusion (NBTC [176]). Liberal transfusion strategies (i.e. transfusing patients above the current recommended thresholds) have not been shown to add any benefit to patient outcomes (Holst et al. [110])
If absolutely required, the patient will safely receive a blood product transfusion without adverse effect or incident.





