Chapter 14: Observations
Skip chapter table of contents and go to main content
Source: Adapted from Marieb and Keller ([111]), Marini and Dries ([112]), Wilkinson et al. ([215]).
Anatomy and physiology
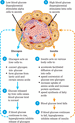
Blood glucose is regulated by insulin and glucagon. Insulin is synthesized and secreted from the beta cells within the islets of Langerhans, which are found in the pancreas (Marieb and Hoehn [110]). It is produced in response to high blood glucose levels, usually after food is consumed, and promotes the uptake and storage of sugar by fat and muscle tissue as glycogen (Wallymahmed [206]). Glucagon is secreted by the alpha cells in response to low blood glucose levels and results in the release of stored sugar back into the blood (Marieb and Hoehn [110]), allowing blood sugar levels to rise (glycogenolysis). Both processes are controlled by a negative feedback system; this is shown in Figure 14.42. In health, these processes maintain blood glucose's stability within the body (homeostasis) (DeFronzo et al. [52]).

Figure 14.42 Negative feedback regulation of the secretion of glucagon and insulin Source: Reproduced from Tortora and Derrickson ([199]) with permission of John Wiley & Sons.
Diabetes mellitus
Diabetes mellitus (DM) is a heterogeneous disorder characterized by chronic hyperglycaemia due to complete insulin deficiency, increased insulin resistance or production of insulin that is inadequate to meet the individual's need (ADA [1]). It is estimated that in 2015, 3.8 million deaths were directly caused worldwide by diabetes and higher‐than‐optimal blood glucose (WHO [214]). This rate is thought to be related to the global increase in the prevalence of people living with diabetes, currently estimated at 422 million and expected to increase to around 629 million by 2045 (WHO [214]).
There are two main types of DM: type 1 and type 2. Type 1 is an autoimmune condition that causes destruction of the beta cells in the pancreas, leading to a complete loss of insulin production (ADA [1]) with known genetic, epidemiological and environmental factors (Phillips [162]). Age was previously believed to be a factor but this is now known not to be the case (NICE [134]). Type 2 is a multifactorial disease characterized by a resistance to insulin that is frequently triggered by poor diet, lifestyle and obesity; however, genetic predisposition has been recognized as a key factor in its development (Phillips [163]). Insulin resistance commences prior to the development of type 2 diabetes (Casey [35]), with the hypersecretion of insulin occurring concurrently (Phillips [163]). This contributes to beta cell exhaustion and lack of insulin production (Casey [35]), which leads to hyperglycaemia (high blood glucose) (Phillips [163]). As the process is frequently much slower than seen in type 1 diabetes, the period of sustained low‐level hyperglycaemia is longer, in some cases years, which allows long‐term complications to occur, such as cardiovascular disease and degenerative changes affecting the kidneys, nerves (neuropathy) and eyes (blindness) (Patel et al. [154], Wilkinson et al. [215]). Further complications of uncontrolled blood glucose include coronary artery and peripheral vascular disease, stroke, and central and peripheral nerve damage, possibly resulting in the need for amputations (NICE [131]).
A diagnosis of diabetes can primarily be based on a fasting blood glucose level of more than or equal to 7 mmol/L or a random plasma glucose of more than or equal to 11.1 mmol/L accompanied by symptoms associated with diabetes such as polydipsia, polyuria and weight loss (NICE [136]). These features of diabetes do not appear until 80% of beta cells are lost; therefore, with increasing insulin resistance being a major driver of type 2 diabetes, changes to lifestyle, diet and exercise have the potential to reverse its development if it is picked up early enough (Van Ommen et al. [202]). However, it must be acknowledged that sustained lifestyle changes require multifactorial components including diet, physical activity, stress management, self‐empowerment and active participation by the individual supported by an appropriate healthcare professional (Van Ommen et al. [202]).
Furthermore, during infection, major surgery or critical illness (such as sepsis, pancreatitis or respiratory distress), counter‐regulatory or stress hormones (adrenaline, noradrenaline, cortisol, growth hormone and glucagon) are released, causing significant metabolic alterations (Rau et al. [168]). These hormones increase insulin resistance, which decreases peripheral uptake of glucose and also promotes glycogenolysis by stimulating glycogen and fat breakdown, causing further hyperglycaemia, also known as stress hyperglycaemia (Palermo et al. [150]). For this reason, patients with diabetes may need additional treatment with insulin or anti‐diabetic medication during acute illness, in order to replicate this homeostasis or improve the body's ability to produce or use insulin (Kotagal et al. [97]).
Hyperglycaemia
Hyperglycaemia can lead to poor clinical outcomes, increased mortality and extended hospital length of stay (Mabrey and Setji [107]). It is defined as random blood glucose of more than 11.1 mmol/L; however, in the absence of symptoms, diagnosis should be established using plasma blood levels and not a random glucose test (NICE [136]). When insulin is deficient or absent, as in type 1 or 2 diabetes, blood glucose levels will remain high after a meal and be raised in times of illness or stress because glucose is unable to enter most cells (Rau et al. [168]). In this way, cells are starved of glucose and the body reacts inappropriately by producing stress hormones that cause glycogenolysis (the breakdown of glycogen to release glucose), lipolysis (the breakdown of stored fat into glycerol and fatty acids) and gluconeogenesis (the conversion of glycerol and amino acids into glucose) (Marieb and Keller [111]).
This causes blood glucose to increase further, which results in a number of signs and symptoms (Kotagal et al. [97]). Water reabsorption in the kidneys becomes inhibited, resulting in frequent, large volumes of urine (polyuria) (Wilkinson et al. [215]). This will cause the person to feel excessive thirst (polydipsia) and may also result in extreme hunger (polyphagia) (NICE [134]). Polyuria and polydipsia will cause dehydration, a fall in blood pressure and electrolyte imbalance (Marieb and Keller [111], Marini and Dries [112]). Moreover, the subsequent loss of sodium (hyponatraemia) and potassium (hypokalaemia) leads to muscle cramps, nausea, vomiting and diarrhoea, confusion, blurred vision, lethargy, cardiac events, coma and eventually death (Wilkinson et al. [215]).
Despite the excessive level of glucose in the body, due to the lack of insulin, the body cannot utilize it effectively, so the body starts to break down its fat and protein stores for energy, which leads to high levels of fatty acids in the blood (lipidaemia) (Marieb and Keller [111]). This can also cause sudden and dramatic weight loss (Tortora and Derrickson [199]). These fatty acids are converted to ketones, which accumulate in the blood more quickly than they can be excreted or used and so cause the blood's pH to fall, resulting in ketoacidosis (Marieb and Keller [111]). Ketones will also be present in the urine. If ketoacidosis is allowed to continue, it can become life threatening, disrupting all physiological processes, including oxygen transportation and heart activity, and causes depression of the nervous system, leading to coma and death (Marieb and Keller [111]). Potential reasons for hyperglycaemia are described in Table 14.16.
Table 14.16 Possible causes of hyperglycaemia and hypoglycaemia
| Hyperglycaemia |
Inadequate doses of insulin
Stress
Infection/sepsis
Surgery
Medications (e.g. steroids)
Variability in oral or nutritional intake
Nutritional support, for example parenteral nutrition or enteral nutrition
Critical illness |
| Hypoglycaemia |
Missed or delayed meals
Not eating enough
Exercise without carbohydrate compensation
Too much glucose‐lowering medication (e.g. insulin)
Excessive alcohol intake
Infection
Muscle and fat depletion (e.g. anorexia)
Diarrhoea and vomiting
Hepatic failure due to tumour or cirrhosis
Adrenal insufficiency
Salicylate poisoning
Insulin‐secreting tumours
Congestive heart failure
Cerebral vascular accident
Concurrent medications (beta blockers, adrenaline)
Surgery |
It has been found that enteral and parenteral feeding contribute to hyperglycaemia both in patients with a diagnosis of diabetes and in those without (Gosmanov and Umpierrez [72]). This is particularly true of patients receiving parenteral nutrition, as it bypasses the gut and therefore the incretin hormones, which help to maintain glucose homeostasis (Gosmanov and Umpierrez [72]). Hyperglycaemia in response to steroids, for example dexamethasone, is another consideration and some researchers believe that steroids’ hypermetabolic action decreases glucose uptake, increases hepatic glucose production and may directly inhibit insulin release (Marieb and Keller [111]). For this reason, these patients will need blood glucose monitoring and may require changes to their insulin treatment or temporary insulin (Wilkinson et al. [215]).
Hypoglycaemia
Hypoglycaemia is a blood glucose level that is unable to meet the metabolic needs of the body (Marini and Dries [112]), normally lower than 4 mmol/L (Adam et al. [2]). It is an acute complication of diabetes that increases morbidity, mortality and the economic cost of diabetes (Kreider et al. [99]).
In healthy individuals, the glucoregulatory system rapidly reduces insulin production and mobilizes energy reserves from the fat and liver to counter hypoglycaemia (Tortora and Derrickson [199]). However, when it does not act promptly, the increasing levels of insulin in the blood cause an abrupt drop of glucose levels (Wilkinson et al. [215]). Often young, healthy individuals can be asymptomatic during episodes of hypoglycaemia, but early symptoms can include sweating, tremor, weakness, nervousness, tachycardia and hypertension (Wilkinson et al. [215]), although these depend not only on the absolute blood glucose but also on its rate of decline (Tortora and Derrickson [199]). Severe hypoglycaemia can lead to mental disorientation, convulsions, unconsciousness and death (Marini and Dries [112]). In addition, research is now suggesting that frequency and severity of hypoglycaemia episodes can cause an increase in diabetic individuals developing dementia in later life (Rhee [176]). Potential reasons for hypoglycaemia are described in Table 14.16.
There are several ways to reduce the risk of hypoglycaemia, including frequent monitoring of blood sugars with home blood glucose tests and occasionally continuous glucose monitoring (Adam et al. [2]). Treatment should ideally consist of the administration of glucose, and the route of administration will depend on the consciousness level of the patient, their treatment and their ability to take oral substances (Marini and Dries [112]). If they can tolerate oral or enteral intake, they should be given a fast‐acting carbohydrate such as 4–5 glucose tablets, 60 mL of glucose juice, 150–200 mL of pure fruit juice or 3–4 teaspoons of sugar dissolved in water (Walden et al. [205]). Drinks such as Lucozade or Ribena are no longer recommended following the ‘sugar tax’ implementation in 2016. Alternatively, give two tubes of 40% glucose gel (e.g. Glucogel) squeezed into the mouth between the teeth and gums (Walden et al. [205]). If the patient is unconscious or the enteral route is inappropriate, give 1 mg of glucagon intramuscularly and repeat capillary blood glucose levels after 10–15 minutes. If the level of blood glucose remains below 4 mmol/L, 10% glucose intravenous administration should be considered, or treatment should be provided as per local policies (Walden et al. [205]). Once blood glucose is above 4 mmol/L and the patient is alert and able to swallow, a long‐acting carbohydrate food should be offered to the patient, such as biscuits, bread or milk (Walden et al. [205]).
Management of type 1 diabetes usually involves administration of multiple doses of insulin through subcutaneous injections. A single episode of hypoglycaemia does not always necessitate omitting insulin; however, if it remains a consistent problem, the treatment should be reviewed and tailored to the patient's current needs (Walden et al. [205]). For optimal control, patients should have access to continuous subcutaneous insulin infusion (CSII) pump therapy (NICE [128], Phillips [162]).





