Chapter 15: Medicines optimization: ensuring quality and safety
Skip chapter table of contents and go to main content
15.21 Inserting an intraosseous needle
Essential equipment
- Personal protective equipment
- Sterile gloves
- Intraosseous needle
- Power driver
- Connector (e.g. EZ‐Connect)
- Dressing pack (e.g. EZ‐Stabilizer)
- Intraosseous needle (e.g. EZ‐IO)
- Syringe (10 mL)
Optional equipment
- Intravenous administration set
Medicinal products
- 0.9% sodium chloride
- Lidocaine 2%
- Chlorhexidine 2% in 70% alcohol sponge
Pre‐procedure
ActionRationale
- 1.
Introduce yourself to the patient or their family, explain and discuss the procedure with them, and gain their consent to proceed.To ensure that the patient feels at ease, understands the procedure and gives their valid consent (NMC [257], C).In a cardiac arrest, it may not be possible to gain consent. E
- 2.Wash hands with bactericidal soap and water or an alcohol‐based handrub, apply an apron and assemble the necessary equipment.
- 3.Assess the patient and the chosen limb to determine what size of intraosseous needle is required (Action figure 3).To ensure that the correct needle is selected (Vizcarra and Clum [360], E).
- 4.Obtain assistance as required.To maintain safety. E
Procedure
- 5.Wash hands with bactericidal soap and water or an alcohol‐based handrub, and apply gloves.
- 6.Take the medication and the prescription chart to the patient. Check the patient's identity by asking them to state their full name and date of birth. If the patient is unable to confirm these details, then check the patient identity band against the prescription chart. If an electronic identity check system for the patient and/or medicine identification is in place, then use it in accordance with hospital policy and procedures. Check the patient's allergy status by asking them or by checking the name band. Note: in an emergency setting, it may not be possible to follow all of these steps.To ensure that the medication is administered to the correct patient and prevent any errors related to drug allergies (NPSA [262], C).
- 7.Inspect the needle set packaging to ensure sterility and then open the sterile pack and all packaging.To ensure the device is safe to use and reduce the risk of complications. E
- 8.Draw up a syringe of 0.9% sodium chloride (10 mL).To prepare for priming. E
- 9.Connect the 10 mL syringe to the connector (e.g. EZ‐Connect) and prime with 0.9% sodium chloride. If lidocaine 2% is indicated, prime the set with the lidocaine.To prepare for administration and remove air from the set. E
- 10.Leave the syringe connected to the connector.To prepare for administration once sited. E
- 11.Connect the needle set to the driver, leaving the needle cap on until ready to insert.To prepare for insertion. E
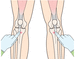
- 12.Palpate the site to locate the appropriate anatomical landmarks for the needle set placement (Action figure 12).To ensure the intraosseous needle is placed in the correct position for optimal drug administration and minimization of tissue damage (Vizcarra and Clum [360], E).
- 13.Select the insertion site. For the proximal tibia, it is approximately 2 cm below the patella and approximately 2 cm (depending on the patient's anatomy) medial to the tibial tuberosity along the flat aspect of the tibia.To ensure the optimal site is used for insertion. E
- 14.Wash hands and apply gloves.To minimize the risk of infection (Fraise and Bradley [98], E).
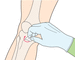
- 15.Clean the site using 2% chlorhexidine in 70% alcohol and allow the site to air dry (Action figure 15).
- 16.Stabilize the insertion site, keeping hand and fingers away from the needle.To guard against unexpected patient movement and avoid a sharps injury. E
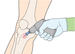
- 17.Remove the needle cap (Action figure 17).To prepare for insertion. E
- 18.Identify the two markings on the needle. The distal mark should be visible once the needle is inserted into the skin. The proximal mark is the maximum insertion point.To ensure that the insertion landmarks are identified. E
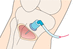
- 19.Insert the intraosseous needle (e.g. EZ‐IO) through the skin at a 90° angle to the bone until the bone is reached (Action figure 19). The distal mark on the needle should be visible. If not, consider using another size of needle or site.To ensure that the needle reaches the medullary space. E
- 20.Press the driver trigger while applying minimal pressure, driving through the bone until a give is heard or felt. Allow the driver to do the work.To ensure that no excess pressure is applied and that the needle is inserted within the bone. E
- 21.Release the driver trigger when the proximal mark on the needle at the hub end is level with the skin.To ensure that the needle reaches the medullary space. E
- 22.Remove the intraosseous needle's power driver from the needle set while stabilizing the needle hub.To prepare for drug or fluid administration. E
- 23.Hold the base of the needle hub with one hand while turning the upper part of the needle anticlockwise.To remove the stylet and prepare for fluid or drug administration. E
- 24.Remove the stylet from the needle (Action figure 24).To prepare the needle for drug or fluid administration. E
- 25.Dispose of the stylet into a sharps container immediately.
- 26.Confirm placement by:
- ensuring the catheter is firmly seated and does not move
- observing blood at the catheter hub
- aspirating marrow from the catheter.
To ensure the needle is in the correct position. E - 27.Apply a suitable dressing (e.g. EZ‐Stabilizer).To secure the intraosseous port and prevent dislodgement. E
- 28.Connect the primed connector to the exposed Luer‐Lok hub and flush with the designated solution (Action figure 28).To ensure patency of the intraosseous needle. E
- 29.Disconnect the 10 mL syringe from the connector.To prepare for drug and/or fluid administration. E
- 30.Connect a primed intravenous administration set or administer bolus drugs.To deliver the prescribed medication or fluids. E
Post‐procedure
- 31.Remove gloves and discard waste, placing it in the correct containers, for example sharps into a designated container.
- 32.Document the time and date of insertion, the size and length of needle, the number of attempts, the location of the device, and the name of the practitioner who inserted the device.
- 33.Assess the site for potential complications or extravasation.To minimize the risk of complications and to treat any complications promptly. E